Abstract
ACKGROUND:
Antiphospholipid syndrome (APS) is defined by arterial and/or venous thrombosis and/or pregnancy morbidity along with persistent circulating antiphospholipid antibodies (aPL). Central to APS pathogenesis are B cells that generate autoreactive antibodies, including anticardiolipin antibodies (aCL), and anti-β2-glycoprotein-I antibodies (anti-B2GPI). Several studies have implicated specific germline and/or antigen-driven somatic hypermutations within the complementarity determining regions (CDRs) of pathogenic antibodies in APS, as well as other thrombotic disorders. Nevertheless, the B cell repertoire in thrombotic APS remains poorly characterized. Recently, autoreactive and platelet activating pathogenic antibodies harboring elongated tyrosine rich CDR3 motifs were reported in patients with heparin-induced thrombocytopenia (HIT) (Zhu et.al, Blood 2019, 2020). Whether such antibodies are present in APS and their potential role, if so, has not been determined.
APPROACH:
We leveraged single cell immunoprofiling to characterize the presence of antibodies harboring elongated tyrosine rich CDR3 motifs in the circulating B cell repertoire obtained from peripheral blood mononuclear cells (PBMC) from patients with thrombotic (n=4) and catastrophic APS (n=3) as well as healthy individuals (n=3). We also correlated the abundance of these antibodies with criteria aPL and inflammatory cytokines in plasma. The modified Ham (mHam) test was used to assess functional complement activation in plasma in plasma from all of the subjects studied (Chaturvedi et al. Blood 2020).
RESULTS:
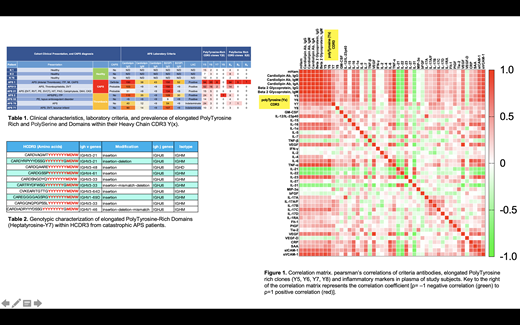
B cell clones containing elongated polytyrosine rich CDR3 motifs were most abundant in patients with a diagnosis of catastrophic APS (n=3) , and were markedly elevated in patients with APS (n=4) compared to healthy counterparts (n=3) (Table 1). Elongated polytyrosine rich CDR3 motifs from CAPS patients contained penta-tyrosine (Y5), hexa-tyrosine (Y6), hepta-tyrosine (Y7), and octa-tyrosine (Y8) motifs (table 2) and contained more tyrosine residues than the penta-tyrosines previously reported in HIT. In addition, all of the polytyrosine containing motifs CDR3 motifs were present on the heavy chain and was followed by MDVW motif of IGHJ6. The presence of B cell clones containing elongated polytyrosine rich CDR3 motifs exhibited significant positive correlations with complement activation measured by mHAM test (r=0.728, p=0.0032), criteria aPLs including anticardiolipin antibodies (aCL) and anti-β2-glycoprotein-I antibodies (anti-B2GPI), as well as plasma inflammatory cytokines; TNFα (r=0.901, p=1.5x10 -5), IL-23 (r=0.780, p=0.0016), IL-17C cytokines (r=0.729, p=0.0033), sICAM1 (r=0.740, p=0.0025), sVCAM1 (r=0.764, p=0.0015), (Figure 1). The presence of elongated polytyrosine rich CDR3 motifs was also associated with the increase abundance of polyserine rich CDR3 motifs that were present predominantly in patients with CAPS.
CONCLUSION:
These preliminary studies provide the first characterization of the prevalence of B cell clones containing elongated polytyrosine rich CDR3 motifs, previously reported in HIT, in the B cell repertoire of APS patients. Notably, the abundance of these B cell clones is markedly elevated in patients with CAPS and is associated with elevated levels of inflammatory cytokines TNFa, IL-23, IL-17C, sICAM1, and sVCAM1. Validation studies in larger cohorts from APS patients and other thrombotic disorders are ongoing.
Chaturvedi: Alexion: Other: Advisory board member; Dova: Other: Advisory board member; Sanofi Genzyme: Other: Advisory board member; Argenx: Other: Advisory board member; UCB: Other: Advisory board participation. Khorana: Bristol Myers Squibb: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Anthos: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Halozyme: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Bayer: Consultancy, Honoraria. McCrae: Sanofi, Novartis, Alexion, and Johnson & Johnson: Consultancy, Honoraria; Dova, Novartis, Rigel, and Sanofi Genzyme: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal